Phenol is often referred to as carbolic acid, and phenolic acid is an aromatic organic compound with the molecular formula C6H5OH. In it, a phenyl group ( -C6H5) is bonded with the hydroxyl group (OH) by a covalent bond. It is acidic in nature and can cause skin burns.
Physical Properties of Phenol
Phenol is a colourless white crystalline solid. Due to the acidic characteristics of phenols, it is often referred to as carbolic acid and phenolic acid. It is a volatile liquid and catches fire quickly. Due to the formation of hydrogen bonding, it is soluble in water.
| Name of the compound | Phenol |
| Molecular Formula | C6H6O |
| Structure | |
| Molecular Weight/Molar Mass | 94.11 gmol -1 |
| Density | 1.07 gcm -3 |
| Melting Point | 40.5 °C |
| Boiling Point | 181.7 °C |
Nomenclature of Phenol
Phenol is an organic compound with a hydroxyl (OH) substituent attached to the phenyl group ( -C6H5). We can classify phenols into three groups based on the number of hydroxyl groups attached to the benzene ring.
1. Monohydric phenol: Monohydric phenol consists of one hydroxyl group. Example: Phenol (C6H5OH).
2. Dihydric phenol: Dihydric phenol consists of two hydroxyl groups. Example: Quinol [C6H4(OH)2].
3. Trihydric phenol: Trihydric phenol consists of three hydroxyl groups. Example: Pyrogallol [C6H3(OH)3].
Synthesis of Phenol
1. Oxidation of benzene and toluene: Benzene and toluene oxidised to form phenol.
C6H6 + O → C6H5OH
C6H5CH3 + 2 O2 → C6H5OH + CO2 + H2O
2. Hydrolysis of benzene sulfonic acid: Benzene sulfonic acid hydrolysed to form phenol. Bayer and Monsanto discovered it.
C6H5SO3H + 2 NaOH → C6H5OH + Na2SO3 + H2O
3. Hydrolysis of chlorobenzene: Chlorobenzene in the presence of an alkali or steam hydrolyse to form phenol.
When the reaction proceeds in the presence of an alkali, it is known as Dow’s process.
C6H5Cl + NaOH → C6H5OH + NaCl
When the reaction proceeds in the presence of steam, it is known as the Raschig Hooker process.
C6H5Cl + H2O → C6H5OH + HCl
Chemical Properties of Phenol
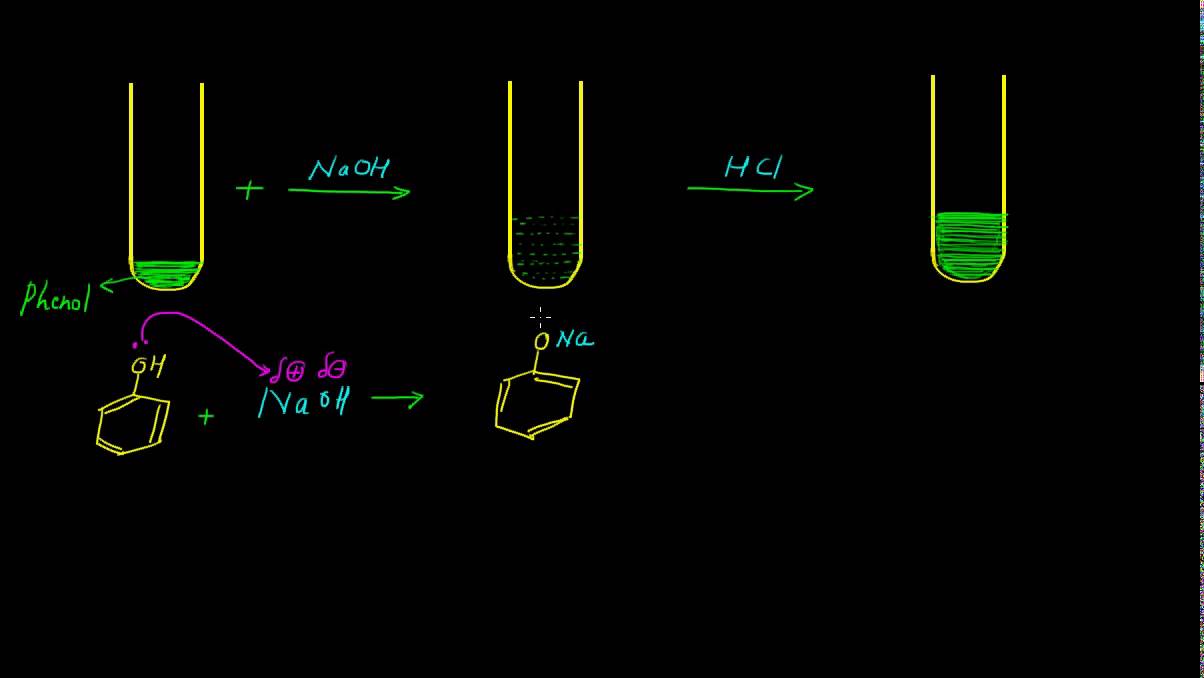
1. Reaction with a base: Phenols are acidic in nature. They react with a base to give a neutralisation reaction to form salt and water.
C6H5OH + NaOH → C6H5Na + H2O
2. Schotten–Baumann reaction: Phenol reacts with an acyl chloride in the presence of a mild alkali to form phenyl benzoate.
C6H5COCl + C6H5OH → C6H5COOC6H5 + HCl
3. Reduction with zinc dust: Phenol gets reduced to benzene when treated with zinc dust at 400 °C.
C6H5OH + Zn → C6H6 + ZnO
Applications of Phenol
Phenol is a universal precursor to an extensive array of drugs. Phenols are used in nylon production and are used as an antiseptic. Phenol is used to preserve vaccines and is used in oral analgesics. It is also used in plastic synthesis and synthesising detergents and carbonates. Derivatives of phenols are used in the cosmetic industry. It is one of the components of the extraction technique used in molecular biology to synthesise nucleic acid. We can also extract DNA and RNA from the body by using phenol.
Phenol is affordable and is used as one of the components of the industrial paint strippers. It is also used in the aviation industry to remove polyurethane, epoxy and other chemical coatings.
Cyclohexanone synthesis from phenol is used as a precursor to synthesise nylon.